Product
Products / rHGH
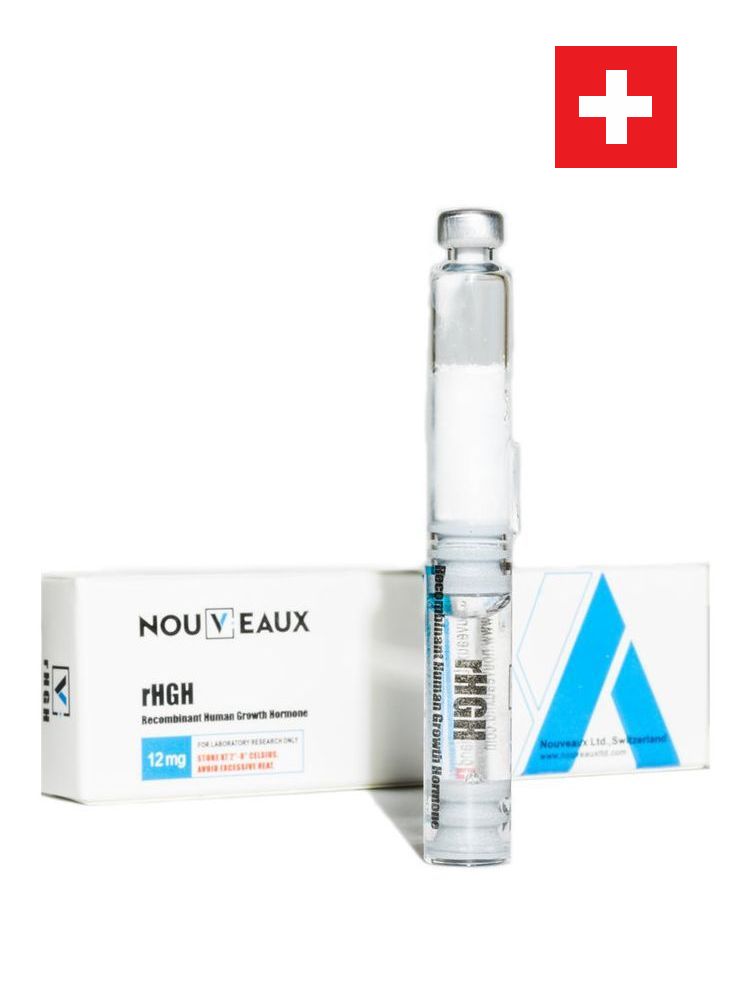
rHGH
Recombinant human growth hormone
12 mg
Recombinant Human Growth Hormone – rDNA origin is human growth hormone produced by identical to the dominant form of this human pituitary growth hormone.
THIS PRODUCT IS FOR RESEARCH PURPOSES ONLY.
+ Description
Nouveaux Recombinant Human Growth Hormone – rDNA origin is human growth hormone produced by identical to the dominant form of this human pituitary growth hormone. It has a molecular weight of 22,125 daltons.
Nouveaux rHGH is a sterile, non-pyrogenic, white lyophilised powder intended for subcutaneous or intramuscular injection, after reconstitution with sterile Water for Injection (0,3% m-Cresol).
+ Mechanism of action
Somatropin (as well as endogenous HGH) binds to a dimeric GH receptor in the cell membrane of target cells resulting in intracellular signal transduction and a host of pharmacodynamic effects. Some of these pharmacodynamic effects are primarily mediated by IGF-I produced in the liver and also locally (e.g., skeletal growth, protein synthesis), while others are primarily a consequence of the direct effects of somatropin (e.g., lipolysis).
- Tissue Growth
- Skeletal Growth
- Cell Growth
- Organ Growth
- Protein Metabolism
- Carbohydrate Metabolism
- Lipid Metabolism
- Mineral Metabolism
- Connective Tissue Metabolism
+ Adverse reaction
The list presents the most serious and/or most frequently observed adverse reactions during treatment with somatropin:
- Sudden death in paediatric patients with Prader-Willi syndrome with risk factors including severe obesity, history of upper airway obstruction or sleep apnea and unidentified respiratory infection.
- Intracranial tumours, in particular meningiomas, in teenagers/young adults treated with radiation to the head as children for a first neoplasm and somatropin.
- Glucose intolerance including impaired glucose tolerance/impaired fasting glucose as well as overt diabetes mellitus.
- Intracranial hypertension.
- Significant diabetic retinopathy.
- Slipped capital femoral epiphysis in paediatric patients.
- Progression of preexisting scoliosis in paediatric patiens.
- Fluid retention manifested by edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias.
- Unmasking of latent central hypothyroidism.
- Injection site reactions/rashes and lipoatrophy (as well as rare generalized hypersensitivity reactions).
- Pancreatitis.
+ Instructions for reconstitution
The injection is given into the sub-cutaneous layer which includes adipose tissue (fat).
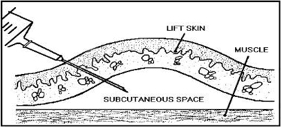
If you are using insulin syringes which have short needles, you will need to enter the skin at 90°. to the skin, otherwise you can inject as shown in the illustration above with a 29 or 30 gauge, 0.5" needle.
+ Dosage
Recommended dosage of rHGH: 2-4 IU once per day.
+ Storage
- This product can be used not more than 3 years from the production date (see box).
- After reconstitution, may be stored for a maximum of 14 days in a refrigerator at 2°C – 8°C.
- Store vials in an upright position.
- Store in a refrigerator (2°C – 8°C). Keep in the outer carton in order to protect from light.
- For one month can be stored at room temperature.